Cis but 2 Ene
The differences between cis and trans isomers can be larger if polar bonds are present as in the 12-dichloroethenesThe cis isomer in this case has a boiling point of 603 C while the trans. The thiol-ene reaction also alkene hydrothiolation is an organic reaction between a thiol and an alkene to form a thioetherThis reaction was first reported in 1905 but it gained prominence in the late 1990s and early 2000s for its feasibility and wide range of applications.

Butene Formula Structure What Are The Isomers Of Butene Video Lesson Transcript Study Com
Periodate Cleavage of 12-Diols.
. Cis-PdCl 2 NH 3 2 trans-PdCl 2 NH 3 2. Due to the polar nature of the bonds in 12-dichloroethylene the boiling point of the cis isomer is 603 o C whereas that of the trans isomer is 475 o C the C-Cl dipole moments. Alpha-Pinene C10H16 CID 6654 - structure chemical names physical and chemical properties classification patents literature biological activities safety.
The terms cis- and trans- are still used but this usage is being phased out. Butene sind unter Standardbedingungen farblose brennbare Gase mit einer größeren Dichte als Luft. --cis-3-acetoxy-4-phenylazetidine-2-one 425-840-9 133066-59-8 - View substance registered dossier.
The existence of discrete inheritable units was first suggested by Gregor Mendel 18221884. This reaction is accepted as a click chemistry reaction given the reactions high yield stereoselectivity high. However cis-and trans-are relative descriptors.
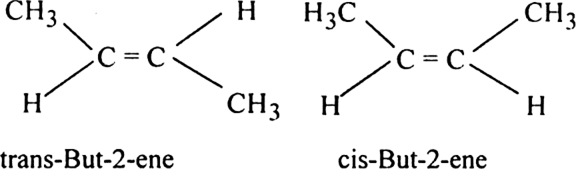
TiGER is a database developed by the Bioinformatics Lab at Wilmer Eye Institute of Johns Hopkins University. 2 Historia Antecedentes Flota Aérea Militar Imperial 1910-1917 Los orígenes de la aviación rusa se remontan a finales del siglo XIX. That is it exists as two geometric isomers cis-but-2-ene Z-but-2-ene and trans-but-2-ene E-but-2-ene.
In plants algae and other. Discovery of discrete inherited units. Alkynes - compounds containing carbon-carbon triple bonds.
It is a petrochemical produced by the catalytic cracking of crude oil or the dimerization. The two methyl groups may be directed on the same side of the double bond cis-or Z or they may be directed away from one another trans- or E. The cis isomer of pent-2-ene has a boiling point of 37 o C but the boiling point of the trans isomer is 36 o The difference is small because the bond polarity is low.
Desde donde se dirige el sistema de defensa aérea común de la CIS. Butene auch Butylene sind eine Gruppe von vier isomeren Kohlenwasserstoffen mit der allgemeinen Summenformel C 4 H 8 die über eine CC-Doppelbindung verfügen. Condizioni Meteo per Dopodomani e per i prossimi giorni a Canazei.
From 1857 to 1864 in Brno Austrian Empire todays Czech Republic he studied inheritance patterns in 8000 common edible pea plants tracking distinct traits from parent to offspringHe described these mathematically as 2 n combinations. Former NONS notifier unknown to ECHA - Contact the relevant French competent national authority. Cieli in prevalenza poco nuvolosi per lintera giornata ma nella notte sono previste precipitazioni.
CSA 7385 7538 7390 7404 SUBSTANCE DEA NUMBER SCH NARC OTHER NAMES 1-Androstenedione 5alpha-androst-1-en-317-dione 4000 III N 1-Methyl-4-phenyl-4-propionoxypiperidine 9661 I Y MPPP synthetic heroin. We would like to show you a description here but the site wont allow us. Although lycopene is chemically a carotene it has no vitamin A activity.
Sides of a double bond. In chemistry a ketone ˈ k iː t oʊ n is a functional group with the structure R 2 CO where R can be a variety of carbon-containing substituentsKetones contain a carbonyl group a carbon-oxygen double bond. -pin-210-ene 242-060-2 18172-67-3 - View substance registered dossier.
Cis-but-2-ene is also known as Z-but-2-ene. En chimie organique on parle disomérie lorsque deux molécules possèdent la même formule brute mais ont des formules développées ou stéréochimiques différentes 1Ces molécules appelées isomères peuvent avoir des propriétés physiques chimiques et biologiques différentes. Sep 07 2022 Typhoon Hinnamnor has left 10 dead two missing and three injured as of 11 am.
Regulation of gene expression or gene regulation includes a wide range of mechanisms that are used by cells to increase or decrease the production of specific gene products protein or RNASophisticated programs of gene expression are widely observed in biology for example to trigger developmental pathways respond to environmental stimuli or adapt to new food sources. CH 3CH 24CH 3 hexane CH 3CH 220 CH 3 docosane CH 3CH 25CH 3 heptane CH 3CH 221 CH 3 tricosane CH 3CH 26CH 3 octane CH 3CH 228 CH 3 triacontane CH 3CH 27CH 3 nonane CH 3CH 229 CH 3 hentriacontane CH 3CH 28CH 3 decane CH 3CH 238 CH 3 tetracontane CH 3CH 29CH 3 undecane CH 3CH 248 CH 3 pentacontane cyclohexane cycloheptane. UltravioletVisible UVVis Spectroscopy.
Tissue-specific Gene Expression and Regulation TiGER. Sie zählen damit zu den AlkenenZwei der Isomere unterscheiden sich durch cis-trans-Isomerie. EZ configuration or the EZ convention is the IUPAC preferred method of describing the absolute stereochemistry of double bonds in organic chemistryIt is an extension of cistrans isomer notation which only describes relative stereochemistry that can be used to describe double bonds having two three or four substituents.
The simplest ketone is acetone R R methyl with the formula CH 3 COCH 3Many ketones are of great importance in biology and in industry. But-2-ene is an acyclic alkene with four carbon atoms. Simple cis and trans isomers may be indicated with a prefixed cis-or trans-.
It is IUPAC convention to describe all alkenes using absolute descriptors of Z-same side and E-opposite with the CahnIngoldPrelog priority rules. You get a mixture of two isomers formed - cis-but-2-ene and trans-but-2-ene. Isomer melting point C boiling point C cis-but-2-ene-139.
It is the simplest alkene exhibiting cistrans-isomerism also known as EZ-isomerism. The database contains tissue-specific gene expression profiles or expressed sequence tag EST data cis-regulatory module CRM data and combinatorial gene regulation data. Syn-dihydroxylation of alkenes OsO 4.
In fact the situation is even more complicated than it looks because but-2-ene exhibits geometric isomerism. ChemTube3D by Nick Greeves is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 20 UK. You can see the same effect with the cis and trans isomers of but-2-ene.
These differences can be very small as in the case of the boiling point of straight-chain alkenes such as pent-2-ene which is 37 C in the cis isomer and 36 C in the trans isomer. Trans-but-2-ene is also known as E. Le terme isomérie vient du grec ίσος isos identique et μερος meros partie.
Wednesday according to an announcement by the Central Disaster Safety Relief Headquarters. Ene-2020 92 recibidos 1 perdido 22 Su-30SM Marina 114 producidos de 137 ordenados para 2024. There must be stronger intermolecular forces between the molecules of the cis isomers than between trans isomers.
Lycopene from the neo-Latin Lycopersicum the tomato species is a bright red carotenoid hydrocarbon found in tomatoes and other red fruits and vegetables such as red carrots watermelons grapefruits and papayasIt is not present in strawberries or cherries. England Wales License. Why is the boiling point of the cis isomers higher.
Typhoon Hinnamnor leaves 10 dead and 2 missing in S Korea Published. The products are but-1-ene CH 2 CHCH 2 CH 3 and but-2-ene CH 3 CHCHCH 3. Aceto France SAS 3 Rue de Genève CS 50363 69451 Lyon Cedex 06 France.

5 2 Geometric Isomers And E Z Naming System Chemistry Libretexts
Which Is More Stable Cis Or Trans 2 Butene Quora

Out Of The Two Trans But 2 Ene And Cis But 1 Ene Which Is More Stable And Why From Chemistry Hydrocarbons Class 11 Cbse

Among Cis But 2 Ene Trans But 2 Ene Which One Is Polar Why Youtube
Comments
Post a Comment